Navigating the Future of Healthcare: Understanding the FDA Drug Approval Calendar for 2025
Related Articles: Navigating the Future of Healthcare: Understanding the FDA Drug Approval Calendar for 2025
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Navigating the Future of Healthcare: Understanding the FDA Drug Approval Calendar for 2025. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Navigating the Future of Healthcare: Understanding the FDA Drug Approval Calendar for 2025
- 2 Introduction
- 3 Navigating the Future of Healthcare: Understanding the FDA Drug Approval Calendar for 2025
- 3.1 Understanding the FDA Drug Approval Process
- 3.2 The Importance of the FDA Drug Approval Calendar for 2025
- 3.3 Key Considerations for the FDA Drug Approval Calendar for 2025
- 3.4 FAQs about the FDA Drug Approval Calendar for 2025
- 3.5 Tips for Staying Informed about the FDA Drug Approval Calendar for 2025
- 3.6 Conclusion
- 4 Closure
Navigating the Future of Healthcare: Understanding the FDA Drug Approval Calendar for 2025

The Food and Drug Administration (FDA) plays a critical role in safeguarding public health by ensuring the safety and efficacy of drugs and medical devices. Its drug approval process, a meticulous evaluation of scientific data, is essential for bringing life-saving therapies to patients. As we approach 2025, understanding the FDA’s drug approval calendar becomes increasingly crucial for stakeholders in the pharmaceutical industry, healthcare professionals, and the general public.
This calendar, though not a definitive list of approved drugs, provides valuable insights into the potential landscape of new treatments and technologies. It serves as a roadmap for the development and commercialization of novel therapies, offering valuable information for investors, researchers, and patients anticipating new options.
Understanding the FDA Drug Approval Process
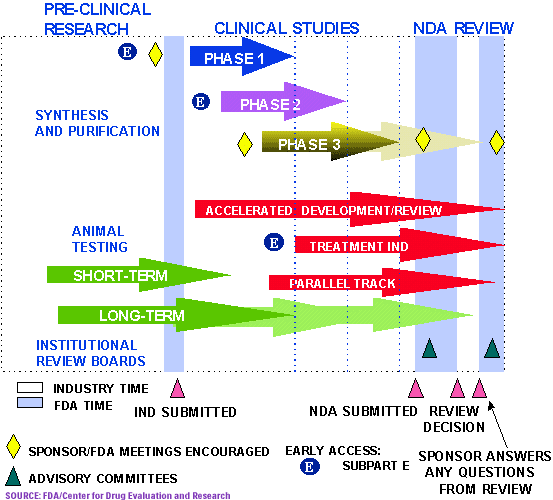
The FDA’s drug approval process is a multi-step procedure that involves rigorous scientific review and assessment. It begins with pre-clinical testing, where potential drugs are evaluated in laboratory settings and animal models. Following successful pre-clinical trials, the drug enters clinical trials, involving human subjects, to assess safety, efficacy, and optimal dosage.
The clinical trials are divided into phases:
- Phase 1: Focuses on safety and dosage in a small group of healthy volunteers.
- Phase 2: Assesses efficacy and safety in a larger group of patients with the target condition.
- Phase 3: Confirms efficacy and safety in a large-scale study, often involving multiple treatment centers and diverse patient populations.
Once the clinical trials are completed, the sponsor submits a New Drug Application (NDA) or a Biologics License Application (BLA) to the FDA, containing comprehensive data from the clinical trials. The FDA reviews the application, including the chemistry, manufacturing, and controls of the drug, as well as the clinical data, to assess its safety, efficacy, and quality.
The FDA’s review process is thorough and can take months, even years, depending on the complexity of the drug and the amount of data submitted. If the FDA approves the application, the drug can be marketed and prescribed to patients.
The Importance of the FDA Drug Approval Calendar for 2025
The FDA Drug Approval Calendar for 2025 offers valuable insights for various stakeholders:
Pharmaceutical Companies: This calendar helps companies anticipate the regulatory landscape and plan their research and development strategies. It provides information on the timelines for approval, allowing companies to adjust their timelines for clinical trials and marketing efforts.
Investors: Investors seeking to invest in pharmaceutical companies can use the calendar to identify potential opportunities. By understanding the pipeline of drugs nearing approval, investors can make informed decisions about their portfolio.
Healthcare Professionals: The calendar helps healthcare professionals stay informed about the latest developments in drug therapy. It allows them to anticipate the availability of new treatments and prepare for their implementation in clinical practice.
Patients: The calendar provides hope for patients seeking new treatments for their conditions. It allows them to track the progress of drugs in development and anticipate potential breakthroughs.
Researchers: Researchers can use the calendar to identify areas of unmet medical need and focus their research efforts on developing drugs with high potential for approval.
The Public: The calendar offers the public transparency into the FDA’s drug approval process, fostering trust and confidence in the regulatory system. It allows the public to stay informed about the latest developments in healthcare and make informed decisions about their health.
Key Considerations for the FDA Drug Approval Calendar for 2025
Several factors influence the FDA Drug Approval Calendar for 2025:
Advancements in Drug Development: Technological advancements in drug discovery and development, including artificial intelligence and machine learning, are accelerating the pace of research and development. This could lead to a higher number of drugs entering the approval process.
Focus on Rare Diseases: The FDA has prioritized the development of drugs for rare diseases, recognizing the significant unmet medical need in this area. This focus is likely to lead to an increase in approvals for orphan drugs.
Personalized Medicine: Personalized medicine, which tailors treatments to individual patients based on their genetic makeup, is gaining momentum. The FDA is actively evaluating personalized therapies and is expected to approve several drugs in this category.
Digital Health and Medical Devices: The rise of digital health and medical devices is creating new opportunities for drug development. The FDA is actively reviewing these technologies and is expected to approve several digital health products in the coming years.
Global Collaboration: Increased global collaboration in drug development and regulatory review is streamlining the approval process. This collaboration is expected to lead to faster approvals for drugs that have the potential to benefit patients worldwide.
FAQs about the FDA Drug Approval Calendar for 2025
Q: Is the FDA Drug Approval Calendar for 2025 a definitive list of approved drugs?
A: No, the calendar is not a definitive list of approved drugs. It is a projection based on the current pipeline of drugs in development and the FDA’s approval process. The actual list of approved drugs may differ from the calendar.
Q: How can I access the FDA Drug Approval Calendar for 2025?
A: The FDA does not publish a specific calendar for a particular year. However, you can stay updated on the latest drug approvals and regulatory updates through the FDA’s website, press releases, and public announcements.
Q: What factors can influence the FDA’s approval timeline?
A: Several factors can influence the FDA’s approval timeline, including the complexity of the drug, the quality and completeness of the data submitted, the availability of resources, and the FDA’s workload.
Q: What are the benefits of a drug being approved by the FDA?
A: FDA approval ensures that a drug has met rigorous scientific standards for safety, efficacy, and quality. This provides assurance to healthcare professionals, patients, and the public that the drug is safe and effective for its intended use.
Q: What are the potential risks of using a drug that is not approved by the FDA?
A: Using a drug that is not approved by the FDA can pose significant risks to your health. These drugs may be unsafe, ineffective, or even harmful.
Tips for Staying Informed about the FDA Drug Approval Calendar for 2025
1. Visit the FDA website: The FDA website is the primary source of information on drug approvals and regulatory updates.
2. Subscribe to FDA news releases: The FDA regularly publishes news releases about drug approvals and other regulatory actions. Subscribe to these releases to stay updated.
3. Follow industry publications: Industry publications such as The New England Journal of Medicine, JAMA, and Nature Medicine often report on drug approvals and regulatory developments.
4. Attend industry conferences: Industry conferences provide opportunities to hear from experts and learn about the latest developments in drug development and regulatory affairs.
5. Network with professionals: Network with professionals in the pharmaceutical industry and healthcare to stay informed about the latest trends and developments.
Conclusion
The FDA Drug Approval Calendar for 2025 offers a glimpse into the future of healthcare, highlighting the potential for new treatments and technologies that could improve patient outcomes. By understanding the FDA’s drug approval process and the factors influencing the calendar, stakeholders can make informed decisions and contribute to the development and delivery of safe and effective therapies. As we move forward, continued vigilance and collaboration are essential to ensure that the FDA’s drug approval process remains robust and continues to serve the public interest.





![]()
Closure
Thus, we hope this article has provided valuable insights into Navigating the Future of Healthcare: Understanding the FDA Drug Approval Calendar for 2025. We hope you find this article informative and beneficial. See you in our next article!